Compressibility factor (Z) for a van der Waals real gas at
5 (187) · $ 6.50 · In stock
Share your videos with friends, family and the world

Deviation of Gas from Ideal Behavior

Solved Real gas effects can be expressed as departures from

Ideal Gas Equation - an overview
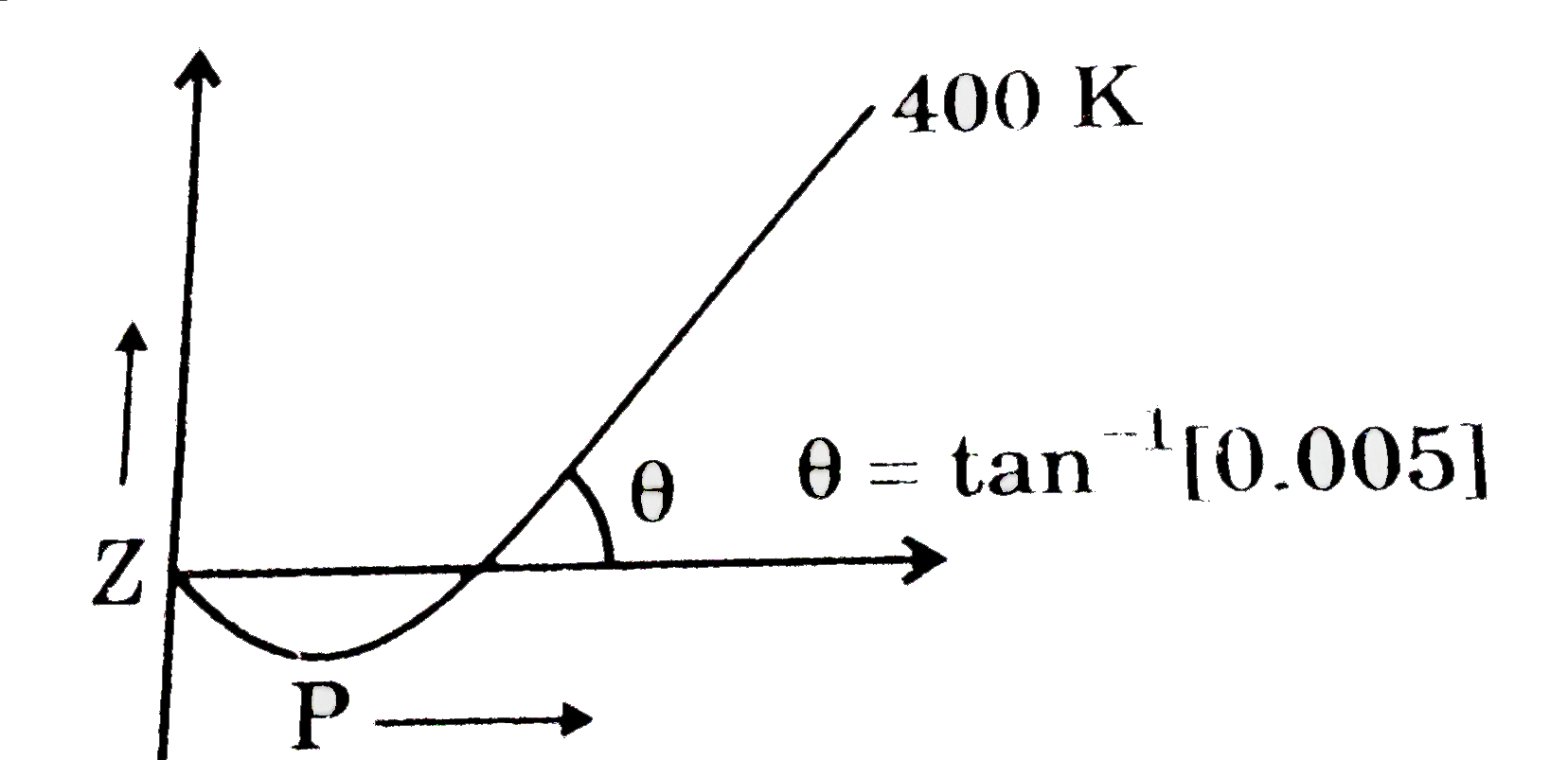
What will be the nature of forces at critical conditions for a real ga

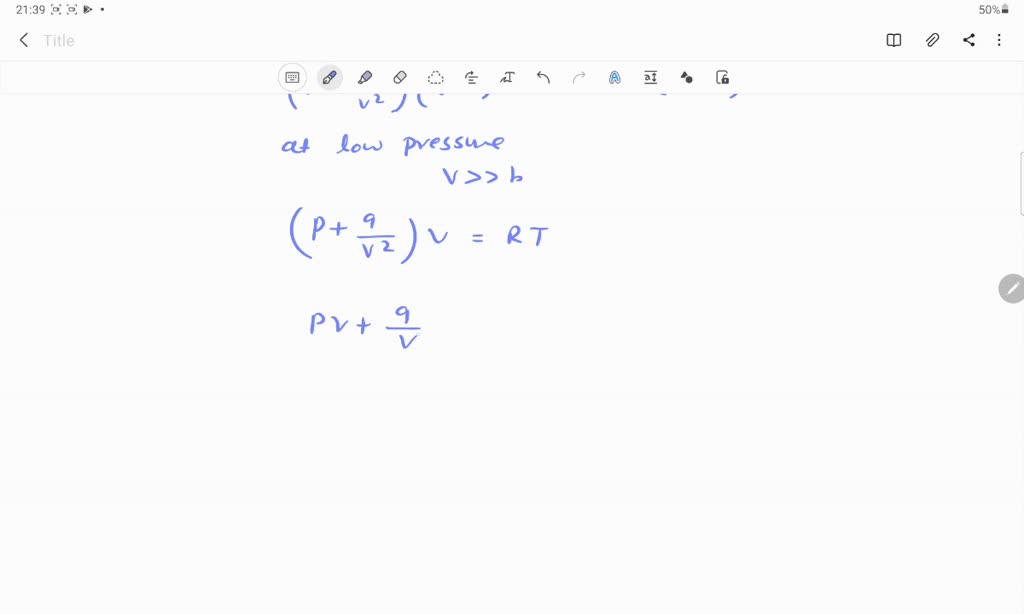
09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very
⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…
Bengali] The compresibility factor (Z) of one mole of a van der waals

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

Real Gases Introductory Chemistry

The compression factor (compressibility factor) for 1 mol of a van der

Physical Chemistry The Compression Factor (Z) [w/1 example]

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt












