What is the compressibility factor (Z) for 0.02 mole of a van der Waal
4.6 (195) · $ 31.50 · In stock
(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

Ratio of the rate of effusion of oxygen gas 1.5 atm to that of helium gas 4.5 atm will be (1) 1:62 (2) 1:12 Yon4 13 (3) 1:22 (4) 1:3 Compressibility factor under critical state of a gas is

6.3: Van der Waals and Other Gases - Physics LibreTexts
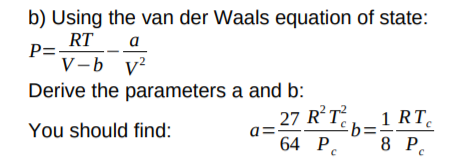
Answered: b) Using the van der Waals equation of…

2. fluids 2
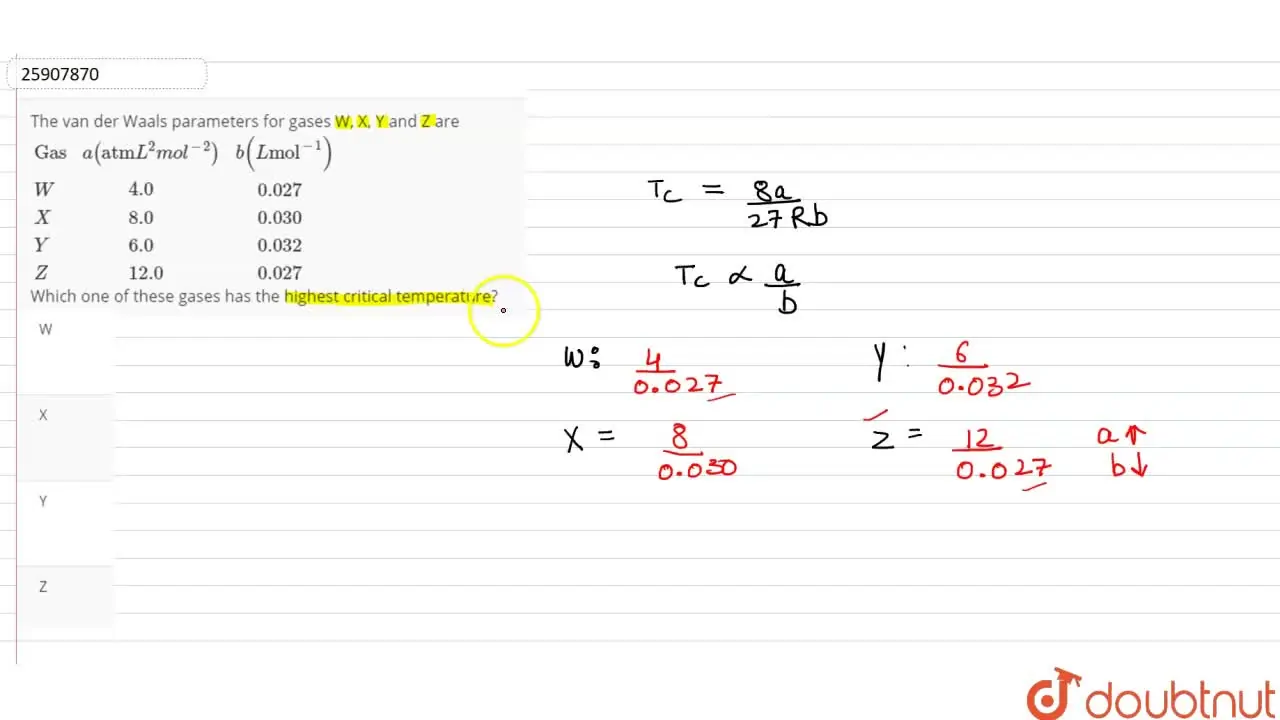
The van der Waals parameters for gases W, X, Y and Z are {:(Gas,a(

At 273K temp, and 9 atm pressure, the compressibility fog a gas is 0.9

Compressibility factor - Wikipedia

Answered: The van der Waals equation of state for…
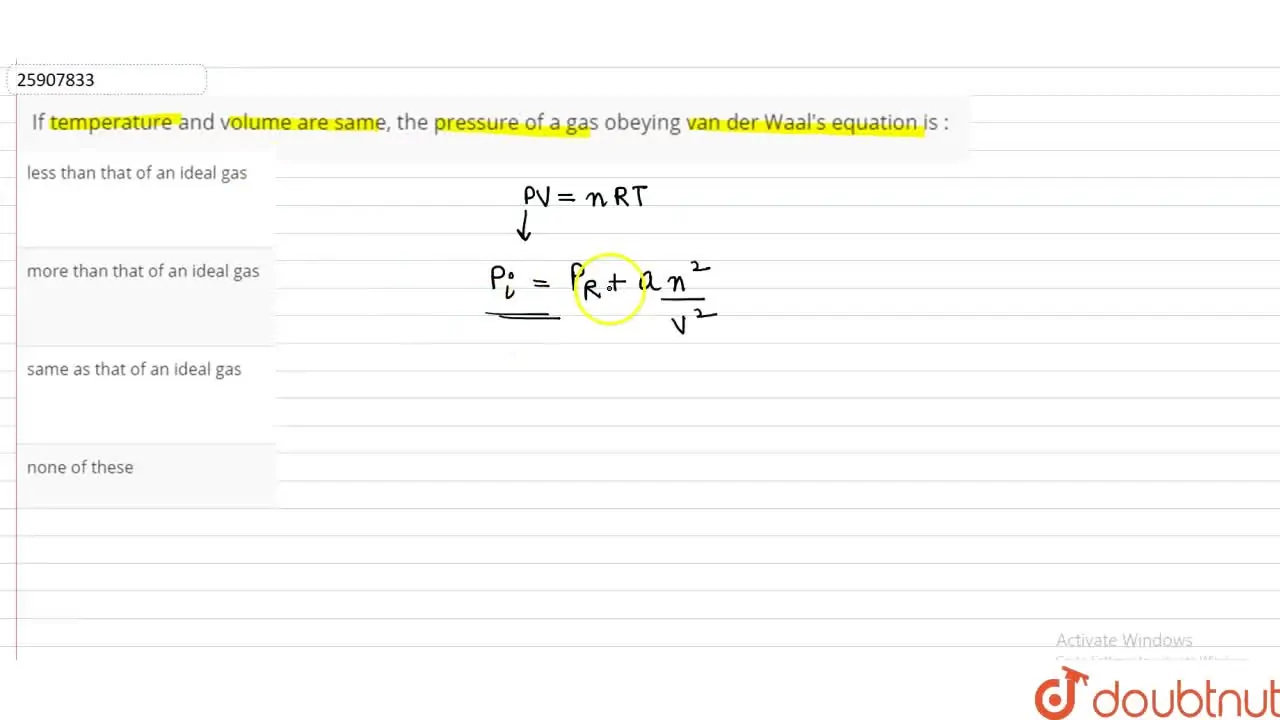
If temperature and volume are same, the pressure of a gas obeying van

100ml of 0.1 M k4 [ Fe (CN)6 ] . Find the molarity of K+












